Understanding the environment through which a sensitive pharmaceutical/life science product must travel is essential for any successful supply chain. It provides the solid foundation for design and choice of packaging that ensures the maintenance of the required product temperature during external distribution.
‘Risk assessment of delivery routes should be used to determine where temperature controls are required.’
Choosing the right route and packaging is one thing – but how well do you know your supply chain partners in terms of their capabilities in Good Distribution Practice standards? Arguably this is just as important since we know that even the best designed and manufactured packaging solution can easily fail if poorly handled by untrained staff who are not aware of the peculiar needs of your shipment. And since the pharmaceutical manufacturing sector is so heavily dependent upon the need to outsource their distribution activities, there has been a clear and welcomed drive by regulators to define the responsibilities of parties in this process:
Any activity covered by the GDP guide that is outsourced should be correctly defined, agreed and controlled in order to avoid misunderstandings, which could affect the integrity of the product. There must be a written contract between the contract giver and the contract acceptor, which clearly establishes the duties of each party.1
Assessing Your Partners
Just like any other risk-based assessment activity, the process starts with a clear and detailed step-by-step analysis of how your product gets to the wholesaler/buyer/finishing plant etc. Depending upon the complexity of the route and nature of the product, using a cross-departmental internal workshop to physically map the variety of handover points is always a useful starting position and often uncovers previously unknown gaps. It almost always has the additional beneficial effect of identifying key risk points and where perhaps contingencies are required as well as establishing a valuable inter-departmental communication process. Using GDP guidance documents such as those from the EU, WHO or PDA, it is possible to compile a ‘checklist‘ of compliance requirements for each respective outsourced supply chain partner against which an audit schedule may be assembled. This can include simpler aspects of clear contractual agreements between partners through:
- Responsibility for pre-conditioning of transportation equipment
- Provision and suitability of temperature-controlled and monitored storage facilities
- Storage facilities have been suitably temperature-mapped
- Ensuring that supplier’s vehicles have been temperature-mapped/qualified
- There are GDP Training programs in place at each supplier with available records
- Use of Quality Management Systems related to GDP
- The provision of risk-based contingency plans by each supplier.
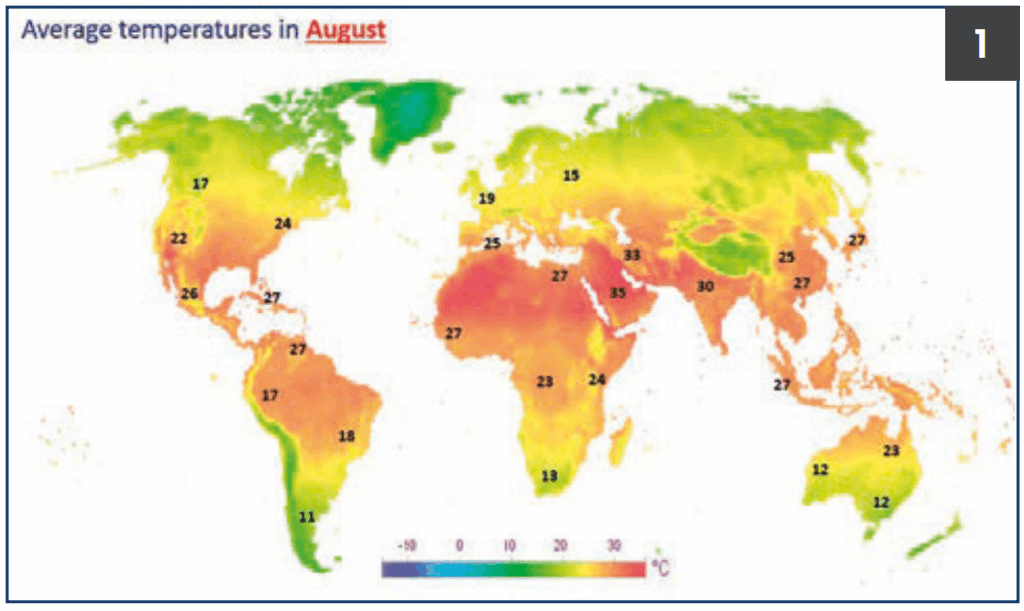
Figure 1.
Average temperatures in August.
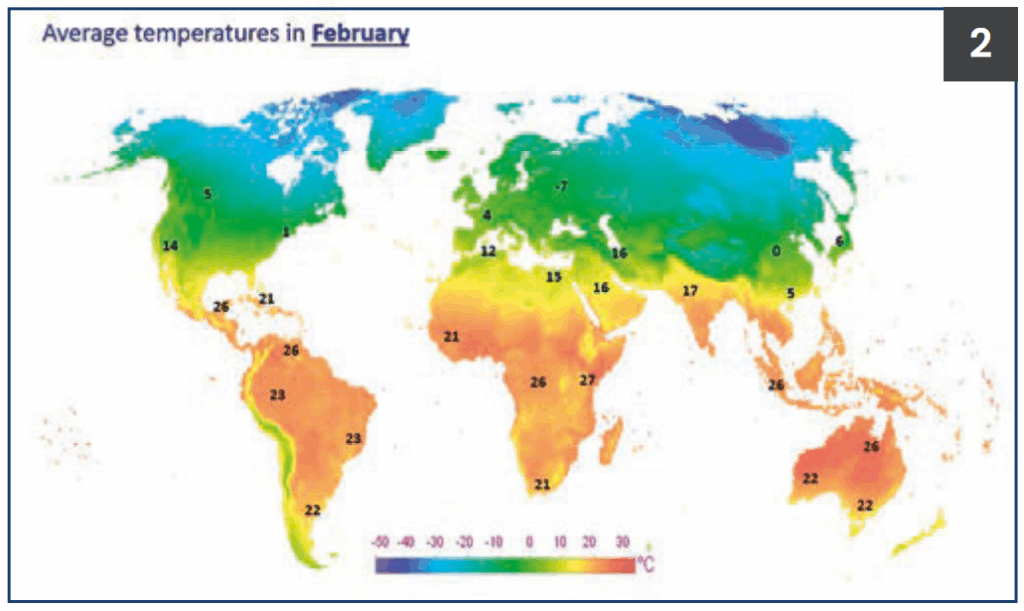
Figure 2.
Average temperatures
in February.
Though this initially requires an investment in time, it represents an essential part of the responsibility for GDP compliance that has been clearly spelled out by the EU:
‘The contract giver is responsible for assessing the competence of the contract acceptor to successfully carry out the work required and for ensuring by means of the contract and through audits that the principles and guidelines of GDP are followed. An audit of the contract acceptor should be performed before commencement of, and whenever there has been a change to, the outsourced activities.’2
Creating this assessment (and audit process) means that changes prompted by alterations in routes, delivery schedules and potentially suppliers can subsequently be more easily managed and GDP compliance continuously maintained.
However, despite a growing appreciation of Good Distribution Practice, evidence shows that there is still a regional variation in its application and within the totality of the supply chain. In recognition of this, CSafe recently worked with one of its key customers to support and guide them through the essential requirements of international GDP and in particular the aspects of outsourced supplier evaluation and continuous performance monitoring.
Through initially hosting a workshop and subsequently creating a working ‘GDP Assessment’ tool that establishes the key supply chain responsibilities within each transportation route, the client has been able to create a GDP audit process against which it can evaluate each of its suppliers. Used for each individual route, the tool identifies each supplier and poses a number of GDP ‘checkpoints’ to be verified and a risk rating for each process stage, together with appropriate corrective actions.
The tool also allows individual and cumulative process timings to be established from ‘start to finish’ that provide a visual understanding of where delays might impact upon the effectiveness of the chosen packaging solution and where contingency plans may be required. With many routes and many individual suppliers to validate, the tool has proved very useful and in the words of the client:
‘The GDP logistics supply chain audit checklist will be highly helpful to strengthen our supply chain.’
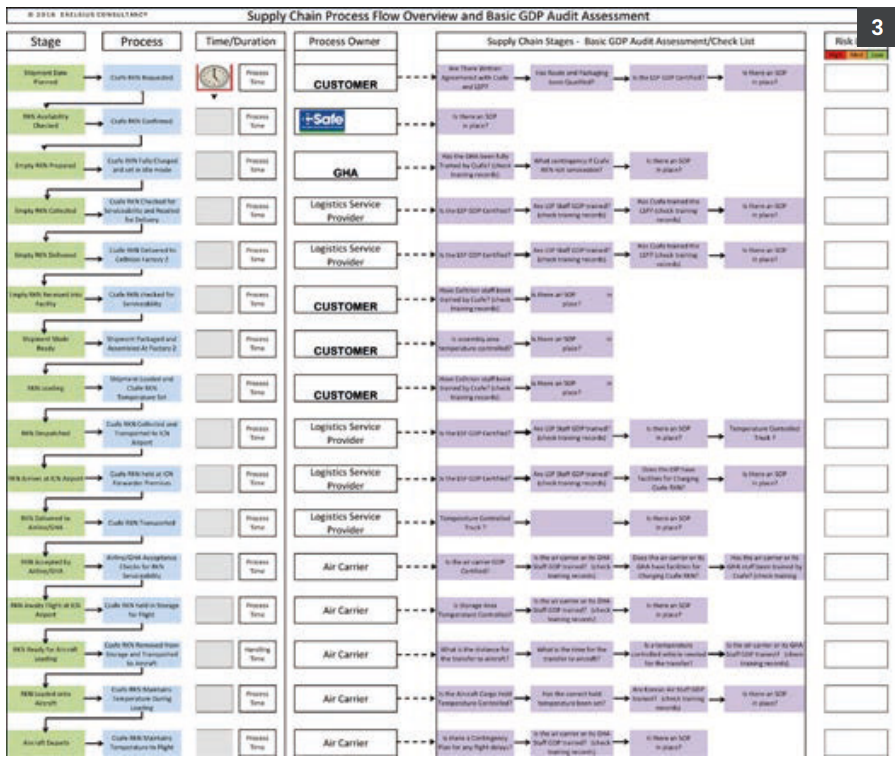
Figure 3.
Courtesy of Tony Wright,
Exelsius Consultancy
Summary
With the largest pharmaceutical-using countries forecast to continue to be pharmerging markets, with two-thirds of the global medicine volumes by 20203, the international manufacturing and distribution of pharmaceuticals will continue to be significant in many ways, including both opportunity and risk.
This example of manufacturer-supplier collaboration is an essential part of the integrity of the supply chain that is at the heart of the enhanced Good Distribution Practice regulations published by the EU, WHO and others who see a contiguous supply chain as a key way forward in improving and maintaining standards for the distribution of pharmaceutical and life science products.
To learn more about how you can benefit from CSafe’s service offering, get in touch.
Your nearest sales representative is ready to help you maximize the impact of your life-saving therapeutics.