ANA Cargo Approves CSafe RKN and RAP Active Air Cargo Containers for Flight
The high-performing temperature-controlled containers from CSafe Global will be available through ANA’s PRIO PHARMA transportation service for pharmaceuticals.
ANA Cargo Inc., the core air freight business of ANA Group, has partnered with CSafe Global, the innovation leader in active, passive parcel and cell and gene temperature-controlled container solutions for the transport of life-enhancing pharmaceuticals, to introduce its high-performing active air cargo containers – the CSafe RKN and RAP – to ANA Cargo’s PRIO PHARMA lineup.
CSafe’s active air cargo units use innovative heating and compressor-driven cooling technologies, along with advanced VIP insulation, to maintain constant payload temperatures even at extreme ambient temperatures spanning from -30°C to +49°C (RKN) and -30°C to +54°C (RAP). The CSafe RAP’s large payload compartment of 6.68m3 easily accommodates up to four standard U.S. pallets or five standard Euro pallets. With an extended battery run times of more than 130 and 120 hours for the CSafe RKN and RAP respectively, CSafe air cargo containers ensure temperature integrity and product viability through to destination even on extended journeys.
ANA Cargo, established in 2013, is the core company of the air freight business in the ANA Group, Japan’s leading airline group, and as of 2019 is ranked 12th in the world transporting 1.15 million tons of air freight volume. ANA is the first Japanese airline to receive IATA CEIV Pharma certification for their reliable transport of pharmaceutical products. Following a vigorous testing process, the carrier has now approved the use of the CSafe RKN and the CSafe RAP on its aircrafts to offer customers continued assurance of compliance and temperature-control.
“We are happy to announce our partnership with CSafe. As the only Japanese IATA CEIV Pharma certified airline since 2017, we have been expanding our capabilities to transport temperature-controlled pharmaceuticals at the global standard and beyond,” noted Norihiko Kurata, Director of Global Marketing at ANA Cargo. “Now more than ever, transporting pharmaceuticals safely is a social mission for us. We want to cater to every customers’ needs various pharmaceuticals including vaccines. The addition of CSafe to our product lineup is our commitment to our valued customers.”
“This new partnership allows CSafe to offer our best-in-class products to ANA Cargo customers to safely transport temperature sensitive pharmaceuticals and biologics,” stated Patrick Schafer, CEO of CSafe Global. “We are thrilled to add ANA as one of our key partners in Japan and look forward to a long and prosperous affiliation.”
ANA Cargo will manage CSafe RKN and CSafe RAP container leases through their PRIO PHARMA service and have provided reservation instructions for customers on their website.
ANA CargoがCSafeのRKN・RAPアクティブタイプの航空貨物用コンテナの使用を承認
CSafe Globalの高性能温度管理コンテナが、ANA Cargoの医薬品輸送サービス「PRIO PHARMA」で利用できます。
2021年1月14日
【デイトン(米オハイオ州)】 ANAグループの航空貨物事業の中核を担う、株式会社ANA Cargoとの提携により、CSafe Globalの高性能アクティブタイプの航空貨物コンテナ(CSafe RKNおよびRAP)が、ANA CargoのPRIO PHARMAのラインナップに追加されました。CSafe Globalは、アクティブタイプ、パッシブタイプの温度管理コンテナや、細胞・遺伝子治療専用のパッケージングなど、生命を豊かにするための医薬品を輸送するためのイノベーションリーダーです。
CSafeのアクティブタイプ航空貨物コンテナは、革新的な加熱システムとコンプレッサー駆動の冷却技術、そして先進的な真空断熱材(VIP)を使用して、-30℃から+49℃(RKN)および-30℃から+54℃(RAP)という極限の外気温度条件下においても貨物の温度を一定に保ちます。CSafe RAPの大きなコンテナ内寸は6.68m3あり、USパレット4枚もしくはEUパレット5枚を容易に収容できます。CSafe RKNは130時間以上、RAPでは120時間以上の長いバッテリー駆動時間を備え、長時間の移動でも目的地までの温度を完全に保ち、製品の安定性を確保します。
ANA Cargoは、日本を代表する航空会社グループであるANAグループの航空貨物事業の中核会社として、2013年に設立されました。2019年現在、航空貨物輸送量は115万トンで、世界12位にランクされています。ANAは、医薬品を輸送する信頼性の高い航空会社として、日本の航空会社で初めてIATA CEIV Pharma認証を取得しました。厳格な検査過程を経て、ANAは、CSafe RKNとCSafe RAPコンテナの同社航空機への使用を承認し、お客様にコンプライアンスと温度管理を常に提供できるようになりました。
「(ANA Cargoは)この度、CSafeとパートナーシップを結びました。2017年以来、日本唯一のIATA CEIV Pharma認定航空会社として、温度管理された環境で医薬品を輸送するための世界的基準や規格以上の機能を拡張しています」とANA Cargoグローバルマーケティング部マーケティング企画課 課長の倉田紀彦氏はコメントしました。「現在、医薬品を安全に輸送することは、弊社の社会的使命となっています。ワクチンを始めとするさまざまな医薬品の輸送を通して、お客様のニーズにお応えしたいと考えています。CSafeを弊社の製品ラインナップに追加したことは、弊社の大切なお客様へのコミットメントを示しています。」
CSafe Global CEOのPatrick Schaferは、「この新しいパートナーシップにより、CSafeは、温度管理の厳しい医薬品やバイオ医薬品を安全に輸送するための、ベストインクラスの製品をANA Cargoのお客様に提供することができます」と述べています。「ANAを日本の重要なパートナーとして迎えることができ光栄に思います。今後の長期的で実り多いパートナーシップに期待を寄せております。」
ANA Cargoは、同社のサービスPRIO PHARMAでCSafe RKNおよびCSafe RAPのコンテナリースを管理
CSafe Global Broadens U.S. Operations Opening a new Hub in Atlanta and Expanding Indianapolis Operation
Partnering with Phoenix Material Management in Indianapolis and Aero Logistics in Atlanta, CSafe continues expanding geographically to meet customer demand.
DAYTON, Ohio, Jan. 19, 2021 – CSafe Global, the innovation leader in temperature-controlled container solutions for the transport of life-enhancing pharmaceuticals, continues expanding geographically to meet customer demand opening a new hub location in Atlanta and significantly expanding operations in Indianapolis.
The new location in Atlanta provides 8,000+ square-feet for storage and operations in partnership with Aero Logistics. “Atlanta is a critical location for international and domestic air cargo shipments and we are pleased to have found a trusted partner to maintain our world-class service standards,” remarked Tom Weir, CSafe COO. The Atlanta hub is fully operational and serving CSafe customers.
In Indianapolis, CSafe has reached an agreement with Phoenix Material Management to offer expanded operations. The new arrangement offers access to 20,000 square-feet of space to service and store CSafe RKN and RAP containers. Weir noted that, “this additional square footage will allow us to better serve key customers in the area and meet increasing demand well into the future.”
CSafe continues expanding its presence worldwide by investing in best-in-class facilities to provide the best service possible to customers in their local area.
THL Completes Sale of 50% Stake in CSafe Global to Frazier Healthcare Partners
BOSTON–CSafe Global (“CSafe” or the “Company”), a leading provider of cold chain shipping solutions to global pharmaceutical and life sciences companies, and portfolio Company of Thomas H. Lee Partners, L.P. (“THL”), has completed the sale of a 50% stake to Frazier Healthcare Partners (“Frazier”).
Headquartered in Dayton, Ohio, CSafe offers a full suite of cold chain shipping solutions and is a leading provider of active air cargo solutions as well as passive parcel and cell and gene solutions. In addition to key acquisitions, CSafe has expanded operations to more than 40 service centers and countless hubs worldwide to ensure product availability and to continue to fulfill its mission to provide patients around the world with access to viable, life-enhancing pharmaceuticals.
“When we invested in CSafe in 2016, we believed in both the quality of CSafe’s offering and the demand for cold chain shipping solutions. Over the past few years, we have worked with management to drive organic growth and operational initiatives, further positioning CSafe for continued success,” said Josh Nelson, Managing Director at THL.
“During the global pandemic, there has been an increased focus on the pharmaceutical supply chain and CSafe has been ready and able to serve its customers despite a challenging operating environment. The Company’s customer-first approach has positioned it well for what we believe to be a critical period for pharmaceutical companies,” said Megan Preiner, Managing Director at THL.
“Over the past few years, THL has been a great partner of ours by helping us further define our business strategy and commercial offering,” said Patrick Schafer, CEO of CSafe. “By adding Frazier as an investor alongside THL, we look forward to the next chapter of value creation for CSafe.”
Together, THL and Frazier will partner with CSafe on the next phase of the Company’s growth. THL and Frazier have a successful history of working together, particularly in pharma services. THL and Frazier will work closely with CSafe to continue to provide best-in-class service to the Company’s growing and valued pharma customers.
“Frazier has been tracking CSafe for several years as a very attractive, highly complementary asset to add to our portfolio. We are thrilled to partner with THL on this opportunity and to back an excellent management team,” said Ben Magnano, Managing Partner at Frazier. “This investment is exemplary of Frazier’s longstanding pharma services thesis, as we partner with Frazier Executive in Residence Bill Mitchell, in his role as Executive Chairman of the Board of CSafe following his successful tenure as CEO of former Frazier portfolio Company PCI Pharma Services.”
Specific terms of the transaction have not been disclosed.
William Blair served as lead financial advisor and Ropes & Gray LLP served as legal counsel to CSafe, and Goodwin Procter LLP served as legal counsel to Frazier.
About Thomas H. Lee Partners, L.P.
Thomas H. Lee Partners, L.P. (“THL”) is a premier private equity firm investing in middle market growth companies, headquartered primarily in North America, exclusively in three sectors: Financial Services, Healthcare and Technology & Business Solutions. We couple our deep sector expertise with dedicated internal operating resources to transform and build great companies of lasting value in partnership with management. Since 1974, we have raised more than $25 billion of equity capital, invested in over 150 companies and completed more than 400 add-on acquisitions representing an aggregate enterprise value at acquisition of over $200 billion. For more information on THL, please visit www.THL.com.
About Frazier Healthcare Partners
Founded in 1991, Frazier Healthcare Partners is a leading middle market private equity firm focused exclusively on healthcare. With more than $4.8 billion total capital raised, Frazier has invested in more than 200 companies with transaction types ranging from buyouts of profitable healthcare services companies to venture capital and Company creation. Frazier has a philosophy of partnering with strong management teams while leveraging its internal operating resources and network to build exceptional companies. Frazier has offices in Seattle, WA, and Menlo Park, CA, and invests broadly across the U.S., Canada, and Europe. For more information about Frazier Healthcare Partners, please visit www.frazierhealthcare.com.
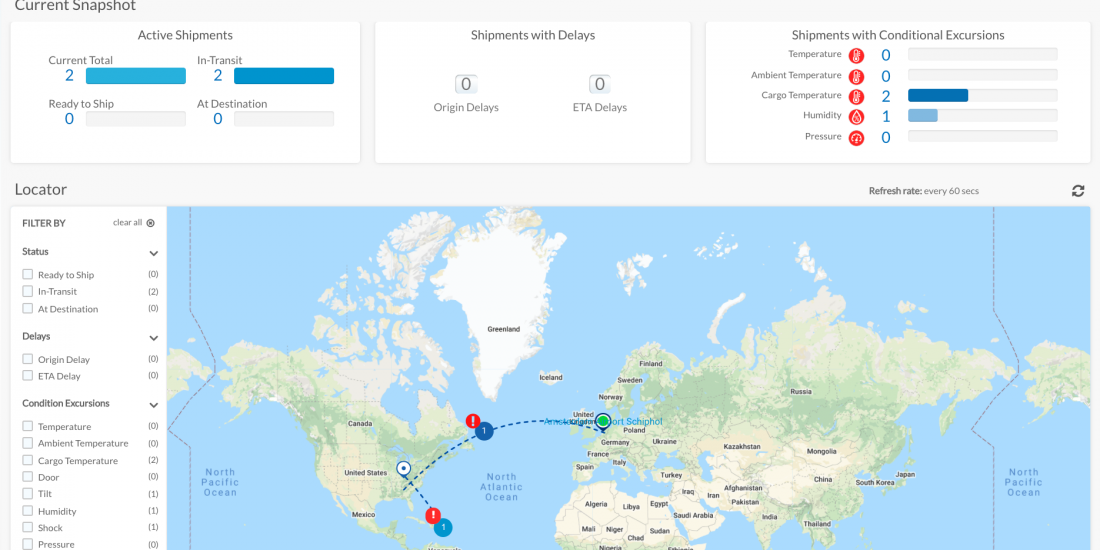
CSafe Global Launches an Industry First: Real-Time Shipment Visibility
CSafe Global continues to focus on innovating temperature-controlled container solutions with the launch of 24/7 real-time shipment visibility.
DAYTON, Ohio, Dec. 1, 2020 – CSafe Global, the innovation leader in temperature-controlled container solutions for the transport of life-enhancing pharmaceuticals, announced today the full commercial launch of the company’s real-time shipment visibility platform and technology that will give CSafe customers the opportunity to make data-driven decisions to ensure the safe delivery of medicines to patients worldwide.
Available now to CSafe air cargo customers, the company has already started retrofitting active RKN and RAP containers with the new sensors capable of tracking location and condition. The platform, custom built by Cloudleaf, Inc. in close collaboration with CSafe operations, provides real-time alerts and notifications directly to customers via email, text or in the platform itself of the following for each shipment:
- Cargo temperature outside set parameters
- Door open events
- Ambient temperature change outside set parameters
- Shock event
- Tilt event
- Ambient pressure change outside set parameters
- Ambient humidity change outside set parameters
- Route deviation / delay
CSafe customers who take advantage of the new platform will also benefit from many other features included with every lease such as:
- Request and manage leases
- Add an unlimited number of platform users
- Monitor all shipments in real-time
- Customize real-time alerts and notifications
- Customize locations for routes and save for future shipments
- Access historical shipments
- Export data in real-time during the shipment to intervene as needed
- Receive GDP compliant standard shipment report
- Use standard rest API packet
“I am beyond proud of the teams of people who have worked tirelessly on this project for more than two years to deliver so much more than just basic functionality to our customers,” said Patrick Schafer, CSafe CEO. “What we have built in partnership with Cloudleaf is a system that is fit for our customers’ needs today and agile enough to easily accommodate enhancements and new innovations well into the future.”
Tom Weir, CSafe COO, who has led the effort from the beginning, noted that, “the entire team is excited about today’s official launch, but that doesn’t mean the work is over. We will be retrofitting the remainder of the active container fleet throughout Q1 before we move on to our roadmap of enhancements. We’ll also be bringing our Cell & Gene customers onto the platform and implementing the system for VIP Parcel containers and customers to offer the capability across our entire product portfolio.”
Weir noted that CSafe has received FAA approval for the tracking device integration into both the CSafe RKN and CSafe RAP active containers.
Proud to Partner in the Effort to Defeat COVID-19
CSafe Global se expande aún más en América Latina con un nuevo centro operativo en la Ciudad de México
CSafe Global se ha asociado con AAACESA en la Ciudad de México para proporcionar contenedores localmente.
DAYTON, Ohio – CSafe Global, líder en innovación en soluciones de contenedores de temperatura controlada para el transporte de productos farmacéuticos que mejoran la vida, continúa expandiendo su alcance global y presencia local abriendo un nuevo centro de operaciones en la Ciudad de México.
Como el segundo mercado farmacéutico más grande de América Latina después de Brasil, añadir este nuevo centro operativo en México a la red mundial de CSafe garantiza un acceso fácil y constante a los contenedores de mejor rendimiento de la industria de la cadena de frío en este mercado en auge. “Estamos encantados de añadir nuestra segunda instalación en América Latina en el 2020”, comentó Tom Weir, director de operaciones de CSafe Global. “El crecimiento del mercado ha sido tremendo en toda la región y esperamos ver un mayor desarrollo a medida que la cadena de suministro farmacéutica comience a diversificar sus operaciones para gestionar mejor la demanda en una pandemia o en cualquier otra situación que se presente en el futuro”.
CSafe se ha asociado con AAACESA en la Ciudad de México para que las unidades de carga aérea estén disponibles en sus instalaciones del Aeropuerto Internacional de la Ciudad de México. CSafe tendrá acceso a 7,000 pies cuadrados (650.32 metros cuadrados) de espacio para almacenar contenedores CSafe RKN y RAP.
CSafe continúa expandiendo su presencia en América Latina invirtiendo en las mejores instalaciones para brindar el mejor servicio posible a clientes en su área local.
CSafe Global Expands Further into Latin America with New Hub Operation in Mexico City
CSafe Global has contracted with AAACESA in Mexico City to provide containers locally.
DAYTON, Ohio – CSafe Global, the innovation leader in temperature-controlled container solutions for the transport of life-enhancing pharmaceuticals, continues expanding their global reach and local presence opening of a new hub operation in Mexico City.
As the second largest pharmaceutical market in Latin America after Brazil, adding this new hub operation facility in Mexico to CSafe’s worldwide network ensures consistent, easy access to the cold chain industry’s top performing containers in this booming market. “We are thrilled to add our second facility in Latin America in 2020,” remarked Tom Weir, chief operating officer for CSafe Global. “The market growth has been tremendous across the region and we expect to see more development as the pharma supply chain begins to diversify their operations to better manage demand in a pandemic or any other situation that comes in the future.”
CSafe has partnered with AAACESA in Mexico City to make air cargo units available at their Mexico City International Airport facility. CSafe will have access to 7,000 square-feet of space to store CSafe RKN and RAP containers.
CSafe continues expanding its presence in Latin America by investing in best-in-class facilities to provide the best service possible to customers in their local area.
Etihad Cargo Approves CSafe RAP Container for Flight
The high-performing temperature-controlled containers from CSafe Global will be introduced across all Etihad Cargo’s flights to deliver additional payload protection for large pharmaceutical shipments
Dayton, Ohio – Etihad Cargo, the cargo and logistics arm of the Etihad Aviation Group, has partnered with CSafe Global, the innovation leader in active, passive parcel and cell and gene temperature-controlled container solutions for the transport of life-enhancing pharmaceuticals, to introduce its latest high-performing container, the CSafe RAP, across its global fleet of wide-body and freighter aircraft.
The CSafe RAP uses innovative heating and compressor-driven cooling technologies, along with advanced VIP insulation, to maintain constant payload temperatures even at extreme ambient temperatures spanning from -30°C to +54°C – the broadest operating range in the industry. Its large payload compartment of 6.68m3 easily accommodates up to four standard U.S. pallets or five standard Euro pallets. With an extended battery run time of more than 120 hours, the CSafe RAP ensures temperature integrity and product viability through to destination even on extended journeys.
Etihad Cargo, which has recently reinforced its pharmaceutical logistics expertise with the launch of PharmaLife, a specialised pharma and healthcare product, was the first carrier in the Middle East to receive IATA’s CEIV (Centre of Excellence for Independent Validators) certification in pharmaceutical logistics. Following a vigorous testing process, the carrier has now approved the use of the CSafe RAP on its aircrafts to offer customers continued assurance of compliance and temperature-control along with the largest payload capacity in the industry.
“As an IATA CEIV Pharma certified carrier, we take the transportation and handling of pharmaceutical shipments very seriously, adhering to the strictest processes with the very best in class equipment that ensures the best quality handling in the quickest time,” said Fabrice Panza, Manager Global Cool Chain Solutions at Etihad Cargo. “The addition of the CSafe RAP further enhances our customer offering by completing our active cool chain portfolio. Through this partnership we are now able to provide the largest and most high-tech solutions of active containers worldwide.
“We are confident as market demands become stronger for vaccines and clinical trials that we are ready to offer quality along with enough capacity to our customers,” Panza added.
“CSafe has the highest quality and best performing products in the industry, as well as the ability to guarantee availability anywhere in the world. We are delighted to be able to offer this new option to Etihad Cargo’s customers,” stated Patrick Schafer, CEO of CSafe Global.
Etihad Cargo has been a long-term partner of CSafe Global and provides customers with a number of closed cool-chain solutions under its PharmaLife product, including the CSafe RAP and CSafe RKN. Sale and leasing options for these containers are available through CSafe Global and Etihad Cargo.
CSafe Global Publishes Results of Second Pilot Test for New Shipment Visibility Capability
CSafe Global has published the results of their second pilot shipment which focused on data accuracy between the real-time data delivered and the data recorded and stored in the containers.
CSafe Global, the innovation leader in temperature-controlled container solutions for the transport of life-enhancing pharmaceuticals, continues rigorous testing of the company’s forthcoming end-to-end shipment visibility capability and has today, published the results of a second key pilot shipment focused on data accuracy.
CSafe is the first cold chain packaging provider to successfully implement end-to-end shipment visibility capability and is nearly through their development timeline. The goal for this most recent pilot shipment completed in August was to determine if container readings, payload readings and pre-established alerts transmitted in real-time during the shipment and matched the validated data the container logged.
“It’s not enough to collect and transmit data,” explained Tom Weir, CSafe’s Chief Operating Officer. “We must be certain the information being supplied to our customers and partners reflects the actual real-time conditions of the container and the payload. Offering complete confidence in the real-time data, provides the assurance our customers need that their products are secure or that intervention is required to preserve a payload. This is what the pharmaceutical industry has been requesting and I am pleased to report that this and subsequent tests have been extremely successful. The hardware and software systems are performing as expected and we are moving into the next phase of testing.”
With more than 20 total pilot test shipments complete, CSafe containers, the integrated tracking devices and the shipment visibility platform have all performed flawlessly. The published results for this test, available in a white paper on the CSafe Global website, provide detailed information on the accuracy of the data collected with the tracking device in the new visibility platform against shipment information collected directly by the containers’ measurement systems. Historically, this information was only available post-shipment.
CSafe published another white paper in July that focused on validating the integration of the tracking device installed in the RKN and RAP containers and the measured parameters tracked.
“The entire project team along with our partners on this project could not be more pleased with these results,” said Weir. “Not only does it confirm that we have chosen the right hardware and software, but also that the planning, effort and investment we’ve made has been well worth it. We are now preparing for the next testing phase and expect similar results.”
Webinar: Trends in Supply Chain Visibility & Implications for the COVID-19 Vaccine Rollout
Event Description
In this final installment of our series on Digital Transformation in the Cold Chain, Tom Weir, CSafe Global Chief Operating Officer and David Parker, Cloudleaf Chief Evangelist will share with you:
- Key trends in supply chain shipment visibility
- How these trends and technology advancements will reshape the cold chain
- The role shipment visibility will play in the COVID-19 vaccine rollout
Registration
Speakers
 Tom Weir leads CSafe’s engineering, product, service, supply chain, manufacturing and quality teams. Mr. Weir brings more than 15 years of executive experience leading global engineering and operations teams. He has developed extensive expertise leading teams in Europe, Ukraine, Mexico, and Asia. His industry expertise includes aviation, cold chain logistics, payment systems, and clinical trial technology. Throughout his career, Mr. Weir has successfully led transformational strategic initiatives in operations, new product development, manufacturing, and service. His professional background includes technical leadership roles at Boeing, United Technologies (Sensitech division), and Crane Payment Systems (formally MEI).
Tom Weir leads CSafe’s engineering, product, service, supply chain, manufacturing and quality teams. Mr. Weir brings more than 15 years of executive experience leading global engineering and operations teams. He has developed extensive expertise leading teams in Europe, Ukraine, Mexico, and Asia. His industry expertise includes aviation, cold chain logistics, payment systems, and clinical trial technology. Throughout his career, Mr. Weir has successfully led transformational strategic initiatives in operations, new product development, manufacturing, and service. His professional background includes technical leadership roles at Boeing, United Technologies (Sensitech division), and Crane Payment Systems (formally MEI).
 David Parker is responsible for promoting the core strategic values of the Cloudleaf offering through sales, marketing and strategic partner engagements. David is an experienced IT professional specializing in business development, operational excellence and sales management. David has held various senior executive positions at Aleri, Sybase, SAP and IBM. He has provided architectural solutions and consultative services for cloud/hybrid deployments of digital transformation solutions for analytics, mobility, ML & AI and data warehouse products for major industries that include Industrial Manufacturing, Oil & Gas, Distribution, Financial Services, CPG, Retail, Telecommunications, Pharmaceutical and Academic institutions.
David Parker is responsible for promoting the core strategic values of the Cloudleaf offering through sales, marketing and strategic partner engagements. David is an experienced IT professional specializing in business development, operational excellence and sales management. David has held various senior executive positions at Aleri, Sybase, SAP and IBM. He has provided architectural solutions and consultative services for cloud/hybrid deployments of digital transformation solutions for analytics, mobility, ML & AI and data warehouse products for major industries that include Industrial Manufacturing, Oil & Gas, Distribution, Financial Services, CPG, Retail, Telecommunications, Pharmaceutical and Academic institutions.