Emilio Frattaruolo, CPP, Vice President of Innovation, Passive Systems discusses how to manage compliance in the cell and gene therapy supply chain.
As with all medical advances and discoveries, cell and gene therapies have seen many ups and downs over time. Today we are in a time of enormous possibility in the field. According to a November 2019 report from Arizton Advisory & Intelligence, the market is expected to grow more than 24% during the 2019-2024 forecast period and reach more than $6.6 billion in revenues. And though there are hundreds or possibly thousands of critical factors to realizing this growth, there is one that can make or break the treatments and the business: supply chain resiliency.
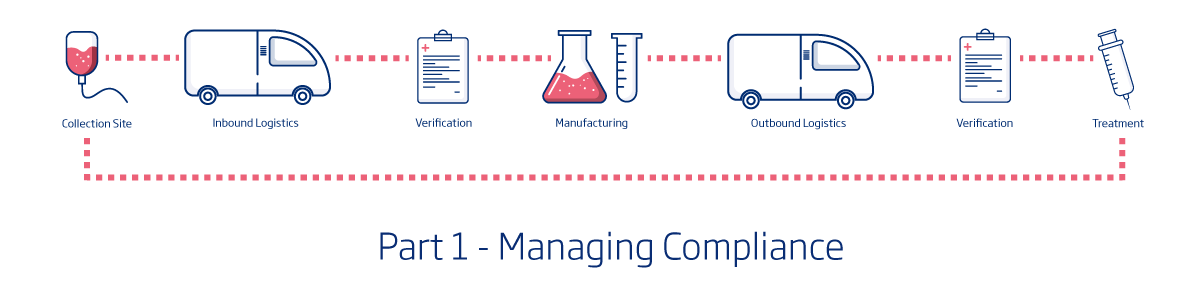
For those not familiar with supply chain resiliency, it’s a fairly simple concept. The image above is a basic supply chain for cell and gene therapies.
For this supply chain to be resilient, it must have the least possible risk associated with it. That means, the collection must go flawlessly and the specimen must be protected until it is opened at the manufacturing facility. The manufacturing must be both efficient and precise and the treatment must again be completely protected until it arrives back at the medical facility for the patient. Any number of things could go wrong in this process. A specimen could be mislabeled, there could be significant weather delays during transport, equipment could malfunction during manufacturing – the list goes on. And in many cases the material is irreplaceable which means there is only one chance to help the patient. With all of the potential risks, how is it possible to truly establish supply chain resiliency?
Luckily, the medical community has addressed the vast majority of the risks associated with collection, manufacturing and treatment through highly regulated processes and adherence to government and industry standards. But what about the transport process? How can we build in safeguards to ensure these life-saving shipments get from point A to point B in perfect condition? The first step is to manage compliance which begins with the box this valuable cargo will go into.
The container that will house any cell and gene therapy for transport should be tested against ISTA (International Safe Transit Association) standards 7D and 3A. . This means that the packaging meets the standards to protect against thermal and physical shock and vibration hazards in the distribution environment.
Once you know you’ve got a packaging solution that complies with the ISTA standards, you have to move on to logistics providers. As you vet potential transportation providers, the regulations become more localized as there are few international standards in place to date. However, if your shipment does need to go by air, the International Air Transport Association (IATA) created the Center of Excellence for Independent Validators in Pharmaceutical Logistics (CEIV Pharma). Their goal is to address the “industry’s need for more safety, security, compliance and efficiency, by the creation of a globally consistent and recognized pharmaceutical product handling certification.” In addition, here are some other regulatory resources for temperature-controlled logistics:
- European Union
- World Health Organization
- Parenteral Drug Association (Technical Report No. 39)
- Pharmacopeia
As I continue with parts 2 and 3 of this series, you’ll begin to realize how critical it is that you have an excellent relationship with every party involved in your supply chain. Transparency, trust and communication among all parties is essential to ensuring your patients get the treatments they need. If you have concerns in any of these areas with any supplier, address those immediately. Because no matter how compliant you and your vendors may be, if there are issues around trust, transparency and communication, something will go wrong one day.