Cold-chain shipping containers: Quality matters
The adage “quality begets quality” — a fundamental underpinning of all mechanically engineered systems — should be top of mind when shipping time- and temperature-sensitive pharmaceutical products. Reliable container performance has direct implications for every stakeholder in the critical chain-of-custody efforts required to efficiently distribute these pharma products across the globe without temperature excursions that can create costly and potentially catastrophic consequences.
Elements of a Quality Container
The overall active container quality directly affects the ability to package, condition, store, ship and deliver temperature-sensitive pharma products and ultimately weather the storms (both actual and proverbial) that will inevitably arise along the route. Reliable, successful delivery of lifesaving therapies and vaccines has both clinical and financial implications. Understanding what goes into building and maintaining a high-quality container is vital for every manufacturer, freight forwarder and airline to maximize reliability and minimize risk.
Container quality begins the moment a new container is initially conceived, however, the key areas where you should focus attention include:
- Overall engineering design
- Raw materials for manufacturing
- Operating principles and procedures
- Continuous improvement
Design for Quality (DFQ)
As previously implied, the design of any active container is essential to long-term quality and performance. Because pharmaceutical products generally have strict temperature requirements over long durations, you should look closely at critical components like the insulation, heating and cooling system, air circulation system and how each affect performance.
Insulating materials come in a variety of forms and compositions. Thanks to steady advances in vacuum insulation panel (VIP) technology, active containers insulated with top-of-the-line VIP provide maximum R-value and minimum thermal conductivity — both measures of peak insulation performance.
We all know air circulation is essential for maintaining a consistent temperature in our homes, vehicles, etc. But it’s even more critical inside an active container that must ensure products arrive at maximum efficacy. Even one hot or cold spot in the container could lead to spoilage of a portion of the payload. The key to eliminating this risk is through optimal temperature mapping and consistent airflow circulation. These two systems work together to control the temperature effectively and reliably inside the cargo space. Containers with optimal temperature sensor placement allow for tight temperature control set-points and broad ambient operating ranges, meeting robust qualification standards. Consistent airflow circulation is required on all sides of the payload. Enveloping the entire payload with conditioned air evenly provides bulk-loading capabilities for maximum payload volume inside the container and long-term savings for all parties.
This combination of advanced design elements allows high-performing temperature-controlled shipping containers to maintain pharmaceutical products within their tightly specified temperature ranges – refrigerated, controlled room temperature, frozen or deep-frozen – during long-duration transit and storage, even in in the face of extreme ambient temperatures.
Managing container quality over time for peak performance
While design and material choices are critical to building a high-quality container, maintaining container performance is equally imperative to meet the stringent requirements for pharma shipments. User operation is yet another component of a successful shipment that requires significant attention. Navigating these elements of quality once a container is in use should not be overlooked.
If a container is difficult to use or your container provider doesn’t offer adequate training for those operating it, you expose your shipments to additional risk of excursion. Everyone involved in the shipment should follow the standard operating procedures (SOP) provided by the container manufacturer and adopt a robust and consistent quality framework and training protocols. Adhering to an SOP is essential to ensure proper and consistent container use, maintenance and troubleshooting. Another hidden benefit — it supports auditing and product-traceability requirements.
Last, but certainly not least, is maintenance. Preventive maintenance is a must if you want to be confident that any container you receive – regardless of age – will perform as well as one newly off the manufacturing line. Your container provider should conduct regular container inspections, annual validation testing and routine preventive maintenance efforts to replace key components preemptively. In this era of heightened environmental responsibility and sustainability, ensuring the longest possible service life also creates an important environmental dividend.
Improving container quality through shipment visibility
End-to-end shipment visibility in active containers for the pharmaceutical cold chain was a long time coming. Now that it’s here, container manufacturers have the opportunity to use the aggregate data to enhance continuous improvement efforts for container quality. Analyzing the data from individual sensors for interior temperature, ambient temperature, shock and tilt, door opening, etc., engineers will gain insights using real world scenarios to improve the quality and performance of existing containers and future fleets.
Ultimately, a high-quality active container significantly reduces excursion risk and can provide a competitive advantage for pharma manufacturers, forwarders and airlines. If your primary mission is to put therapies and vaccines into the hands of prescribers and patients at maximum efficacy, remember – quality begets quality.
Air Cargo Shipment Data in Real-time: Access Options for Every Stakeholder
When it comes to shipping temperature-sensitive pharmaceutical products, there is little room for error. Throughout the journey, everything must go as planned, and — in an ideal scenario — any deviation must be addressed quickly, so that the most appropriate, timely and cost-effective response or intervention can be employed.
The relationship between pharmaceutical and life sciences companies, their shipping partners (including specialized freight forwarders and the airlines) and thermal packaging providers is critical. It requires intense collaboration and transparency to ensure each shipment meets regulatory requirements and that patients have the peace of mind that their medicine or vaccine is as effective as it was when it was manufactured. This is where shipment visibility capability can play a key role in the relationship and collaborative efforts between these various stakeholders in the cold chain. Tracking shipments in real-time allows all those involved in a shipment to receive rich, data-driven insights and critical alerts whenever a parameter varies outside set ranges to allow action to preserve payloads.
CSafe Global’s Air Cargo container fleet is currently being equipped with monitoring devices that provide real-time tracking and visibility related to the critical attributes below. Because the temperature-controlled air cargo container is the only element of the cold chain involved in the shipment from end-to-end, it only makes sense for the container provider to offer the capability.
But true visibility is about more than the device. The data must be easily accessible to all the stakeholders without creating more work. To accomplish this task, CSafe offers two ways to access the data.
Access option 1: Direct platform login
Accessing the data directly in the provider’s platform is likely the simplest solution for most shippers. Often available through a web portal or a mobile app, a user should have easy access to this data in the form of dashboards and detailed reporting. Of course, if the shipper is not the container lessee, providing login access should always be the lessee’s decision. That means regardless of how the lessee accesses the data, they would have to enable access for the shipper to see the data. The shipper would receive condition alerts and be able to track the shipment in real-time and download GDP compliant shipment reports. Available reports for download include:
- Shipment summary report which includes the shipment timeline, containers on the shipment, temperature profile and route information
- Container reports, which includes condition analytics and a full data log for each container on the shipment.
- Additional data/analytics are available in the visibility platform to monitor overall shipment trends.
Access option 2: Lessee platform interface
Using REST API (application program interface), customers who lease containers can have a direct connection to the platform that will constantly deliver shipment data into their transport or incident management system, or other platform used for managing shipments. Automatic flow of critical, timely information reduces the risk of human error related to manual data entry or visually cross-referencing system data. This is especially important for freight forwarders, airlines and pharmaceutical companies managing hundreds or thousands of shipments. Similarly, when shipment visibility data is integrated into company’s incident-management system, it can trigger not just an alert or alarm, but an automatic action, as well, thereby saving critical response time.
Which option is best for your organization?
For the lessee who has a shipment management system in place, the decision of whether or not to integrate the CSafe Global visibility platform via the API is not necessarily black-and-white and it can evolve over time. For instance, if a freight forwarder is only handling a couple of shipments/month, it may not make sense to use up technical resources to carry out the integration. Or, the lessee’s existing system may not provide the same state-of-the-art, highly visual, user-friendly experience offered in the container provider’s platform.
Similarly, the web portal makes it easy for users to access reports related to both the overall shipment and individual container to meet regulatory requirements. Through the API connection, the user can easily access all of the data, but (depending on what system the lessee uses), may not necessarily be able to produce a validated report that is sufficient for regulatory review.
For the shipper who is not the lessee, the decision is far simpler. Once your chosen freight forwarder or airline is setup in the container provider’s system whether directly or via API, they would simply provide you access to view your shipment data in the container provider’s platform. Again, this is a simple action in the CSafe shipment visibility platform and will allow you as the shipper to view real-time shipments and have immediate access to GDP compliant shipment reports.
Using the real-time data for optimal outcomes
Building data-visibility goals and requirements into Standard Operating Procedures (SOP) also provides a framework that enables continued dialogue among all parties. This helps to ensure that the process is working — and enables ongoing process improvement, as well, if it is not.
The more stakeholders can track the movements and the status of all temperature-controlled shipments throughout their cold chain journey, the more they can reduce product losses and the financial penalties associated with them. Having continuous visibility, actionable insight and accurate, real-time analytics gives pharmaceutical and life sciences companies — and the supply chain partners they have enlisted — peace of mind that their shipment will arrive on time in the proper condition, with minimal delays and product losses. At the end of the day, this collective effort ensures that patients get what they need.
Ensuring GDP Compliance with Shipment Visibility
Across the pharmaceutical and healthcare landscape, an increasing volume of approved pharmaceutical products must be temperature controlled from the moment they are manufactured until shortly before they are administered. Such products create high stakes for all partners in the complex supply chain, which must now be entrusted to keep the fragile products within their specified temperature range — refrigerated (2 to 8°C), controlled room temperature (15 to 25°C), frozen (-20 to -80°C) and deep frozen (-150°C and colder) — no matter what may arise during storage and transit. Today, such high-value, temperature-sensitive therapies include oncology medications, biologic therapeutic agents, novel cell and gene therapies (CGTs), vaccines (the COVID-19 vaccine and others) and more.
With any temperature-sensitive pharmaceutical shipment, plenty can go wrong along the way. If travel delays and other complications aren’t addressed quickly, these therapies are at risk of experiencing temperature excursions that could lead to reduced efficacy resulting in spoilage and discard. Spoilage has significant financial consequences for drug manufacturers and potential life-threatening consequences for patients who aren’t able to receive their treatments at the prescribed time.
Unfortunately, there is no globally adopted regulatory framework in place today to govern uniform practices for partners in the pharma cold chain. Instead, participants typically rely on a patchwork of voluntary industry guidelines and best practices.
Good Distribution Practice (GDP) provides an internationally accepted quality system that helps create greater uniformity of policies and protocols related to product packaging, shipping, storage and transportation, with the goal of ensuring the integrity of temperature-sensitive pharmaceutical products and reducing risk.
Overall, the GDP guidelines serve two purposes:
- To codify practices needed to optimize the packaging and transportation of temperature-sensitive products and ensure they remain fit for human consumption.
- To provide a systematic framework to meet the needs of a regulatory audit.
Key Factors for GDP Compliant Shipments
Adherence to GDP guidance can help pharmaceutical manufacturers ensure that every patient receives their treatment and ultimately reduce the overall cost of manufacturing operations as loss due to spoilage could be drastically reduced. There are five key elements of the GDP guidance critical to preserving therapeutic efficacy. Shippers can now definitively meet each of these guidelines for air cargo shipments with real-time shipment visibility.
Product traceability
Real-time GPS location tracking allows all parties involved in a shipment – the pharmaceutical manufacturer, airline or freight forwarder – immediate insight to the shipment’s position throughout the entire journey and provides details of any route deviations that may create unsustainable delays. Armed with this information, both parties have the opportunity to intervene to preserve the shipment and prevent spoilage.
Product efficacy
Temperature monitoring devices integrated into the air cargo container allow customers to receive real-time alerts when either cargo or ambient temperature deviates from the set threshold for a specific shipment. Stakeholders can again, intervene if needed to resolve the issue or easily confirm that it was a temporary issue and no product excursion occurred allowing the therapies to continue the journey and be safely used.
Product safety
In addition to ensuring that the product shipment remains within its setpoint temperature window, air cargo containers equipped with sensors on the doors and linked to a shipment visibility platform can offer real-time alerts when doors are opened or closed (and for how long). This provides important insight to help reduce not only temperature excursions but other potential threats to these therapies.
Damage prevention
The ability to detect and capture data related to shock and tilt events, and create related alerts, provides insight into how the products are being handled during transit, and capture a history of events that may be needed, should the product arrive in a damaged condition and require an assessment.
Compliance record
With such rigorous data collection throughout the journey, and the ability for all stakeholders to always have in-depth shipment visibility, shippers and their pharmaceutical company partners are able to receive critical information in real-time and create detailed GDP-compliant reports at any point along the container’s route.
Beneficial Side-Effects of GDP Compliance
GDP guidelines are in place to protect pharmaceuticals so they arrive to the patient at maximum efficacy. If this happens with 95% of shipments, the cost to distribute pharmaceuticals will go down drastically because so much less will be lost to spoilage.
For pharmaceutical manufacturers, this means selling those therapies rather than losing the funds invested. For freight forwarders, airlines and warehouses, happier customers and ideally more shipments of more pharmaceuticals. For patients, a reliable supply chain to receive medicines and vaccines on time and the peace of mind that it is in perfect condition.
While spoilage risk can never be completely eliminated, with shipment visibility supporting GDP compliance, shippers can more easily make efforts to reduce it. Doing so will ultimately strengthen the cold chain and safeguard high-value, temperature-sensitive therapies and provide peace of mind for everyone from the manufacturer to the patient.
CSafe Global Shipment Visibility
CSafe launched the first real-time shipment visibility for air cargo containers in December 2020. We are currently retrofitting our entire fleet with tracking devices that allow the location and condition of each container to be tracked in real-time via a cloud-based platform.
Battery Performance Offers Insight into Active Container Quality
As the world awaits a COVID-19 vaccine, the cold chain industry is doing everything possible to prepare for the eventual distribution effort. This is no small task and it will require cooperation on levels we’ve never imagined. With all that on our plate, why am I talking to you about batteries? It’s a good question and the answer is simple: it’s not really about the battery.
Isn’t a battery just a battery?
Battery performance isn’t something we often discuss in terms of innovation in the cold chain. Batteries are a critical component of any active temperature-controlled container, but a battery is a battery, right? Well, yes and no. Batteries are designed and manufactured to meet every demand you might imagine. However, battery performance can vary. A pharmaceutical shipper can take identical two batteries, install them in two different active containers, send those containers out on a shipment under identical conditions and have very different battery performance results. Why? The answer lies in the container design and technology.
Testing the theory
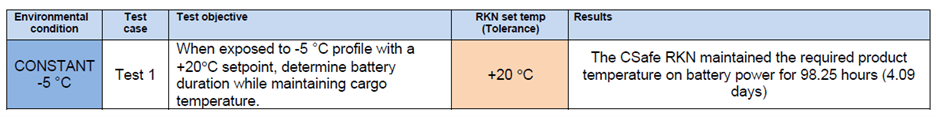
A couple of months ago a customer contacted us and asked our engineers to test the CSafe RKN according very specific parameters and document the battery performance. We of course agreed and conducted the test in our ISTA Certified Testing Laboratory.
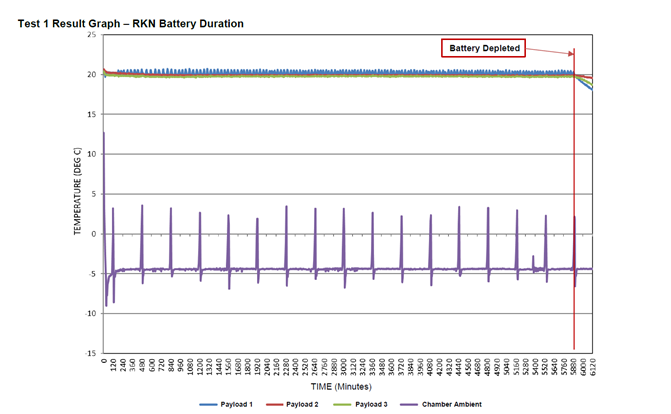
The team loaded a CSafe RKN cargo compartment with a preconditioned payload of 60kg of bottled water and configured the container to maintain the payload temperature at +20 °C. They set the temperature in the test chamber to a constant -5 °C and monitored the battery performance. Once the test was complete and the data was documented, we contacted the customer to share the results that under those conditions, the battery run time was more than 98 hours.
 Are the results unusual?
Are the results unusual?
That’s exactly what we asked the customer when they reacted with surprise at these results. Battery performance does vary based the thermal profile a payload requires and CSafe containers have exceeded 120 hours with other customer specific combinations of payload and ambient profiles. But the solution this customer had been using for some of their shipments could only maintain power for 25-30 hours under the same conditions used in our test. Now, the batteries in the CSafe containers and this other solution are not identical, but in theory should perform equally well. Which is why the difference in run time provides key insights into overall container quality and system autonomy.
How container design drives improved battery performance
Insulation, temperature management and air circulation are the key design features in CSafe containers that directly affect system autonomy and extend battery duration.
Insulation
A container’s insulation allows it to use passive energy to maintain the desired temperature thereby reducing the drain on the battery. Vacuum Insulated Panel (VIP) insulation provides 10 times greater thermal efficiency than standard foam insulation. CSafe containers use the highest quality VIP available and provide the longest hold over time in the industry – up to 6 times better than the industry standard. The VIP in CSafe containers provides the best maximum and minimum ambient temperature operation with no limitations which further reduces the impact on the payload to extend the battery run time.
In addition to preserving battery life, this passive holdover performance allows ample time to react and preserve the payload in the event of a power outage or lack of power supply.
Temperature Management
This is the system within an active container that monitors the payload temperature and automatically adjusts the heating and cooling systems to maintain the required temperature. Many systems do this by monitoring outflow air temperatures. The more often the heating and cooling system is used the larger the drain on the battery. This is why CSafe containers use a full array of 10 sensors in the CSafe RAP (six in the RKN) to monitor zone locations throughout the payload compartment and most importantly the return airflow to precisely regulate payload temperatures. The additional monitoring allows for tighter temperature tolerances and reduced battery usage further prolonging battery life.
Airflow System
Maintaining uniform temperature throughout a container is dependent on regulation by the airflow system. An airflow system has the potential to develop hot or cold zones based on the design. These hot or cold zones would force the temperature management system to overcompensate requiring additional battery power.
CSafe containers use an innovative air recirculation system that fully encapsulates the payload with controlled, conditioned air on all sides. This provides accurate temperature mapping, creates uniform temperature throughout the container and prevents ambient temperature convection especially when sitting on a hot tarmac. Preventing these hot and cold zones allows the container to better manage the payload temperature and preserve the battery life.
Maintenance
While it has nothing to do with design, container maintenance is just as important in overall battery performance. Testing batteries on a regular schedule is standard for container maintenance. However, after a certain time period even if a battery passes a performance test, the likelihood that it could begin to degrade prior to another test increases. This is why CSafe replaces container batteries every three years as part of our Preventive Maintenance Rebuild program.
Final Thoughts
In a world that is experiencing dramatic constraints, unprecedented uncertainty and variability, extended operation on battery power is more important than ever. We now know that shippers must be prepared to safely move their products accounting for risks related to a pandemic, major political shifts like Brexit and long-term trade wars on top of record-breaking hurricane seasons and a plethora of other natural disasters that can impede air travel.
As we prepare for the monolithic task ahead to distribute a COVID-19 vaccine, air cargo container battery performance should be top of mind for every pharmaceutical manufacturer – whether or not the product inside the container is a COVID-19 vaccine. All medications will need to continue to move around the world and until the pandemic nears an end, the transportation challenges we’re seeing now won’t ease by much.
The Fundamentals of Extracting Data from the Cold Chain
Innovation comes in many forms and when it comes to extracting data from the cold chain for customers. Tom Weir, CSafe COO shares insights on innovating with fundamentals in mind.
Continue ReadingArtificial Intelligence Finally Goes Cold
Tom Weir, Chief Operating Officer for CSafe Global discusses why it has taken so long for AI to come to the cold chain industry and what it means now that it’s arrived.
Continue Reading