This year, thousands of cell and gene therapy clinical trials are running around the world. Some of those studies have yielded treatments for blood cancers, solid tumors, and genetic disorders that are so promising, they are poised for regulatory approval in the next 12 months.
But if we can’t actually deliver these transformative clinical trials and therapies to patients, little of that scientific progress will make the difference that it should.
In 2023, more than ever, cell and gene therapy (CGT) needs growth in enabling infrastructure. After two of the sector’s biggest annual events, held back-to-back in January, it’s increasingly clear that the CGT supply chain needs to be as innovative as the therapies themselves.
At the first big CGT-related event of the year, the J.P. Morgan Healthcare Conference, the Alliance for Regenerative Medicine presented a compelling set of data that showed continued growth, even amid economic and investment challenges. According to ARM, 2,220 clinical trials are operating around the world, with 60 percent of those studies focusing on oncology. Almost the same number of trials have a potential application in a prevalent disorder. More than 1,450 CGT developers are working worldwide, up 11 percent from last year.
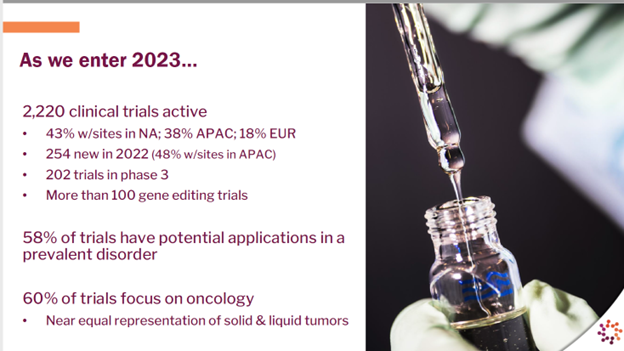
– Alliance for Regenerative Medicine 2023
ARM also anticipates 18 U.S. or EU regulatory decisions in the coming year, across a range of cancers and genetic disorders. Making those approvals viable, though, will require thinking as much about access as the science. That means modernizing all aspects of the infrastructure that supports CGT, from systems within hospitals to payment mechanisms to the biopharmaceutical cold chain. As ARM put it succinctly, this is “an opportunity we can’t miss.”
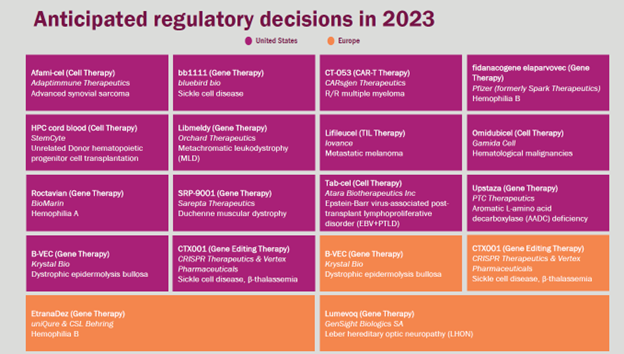
– Alliance for Regenerative Medicine 2023
Thankfully, when it comes to cold chain, the CGT sector has proven practices to learn from. While CGTs present the most complex supply chains in the history of medicine, a set of baseline requirements has emerged across product categories and temperature ranges, including:
- Ensuring just-in-time cellular material and final product distribution.
- Protecting product quality, even when supply chains experience unexpected changes mid-process.
- Enabling appropriate product handling.
- Safeguarding product security against tampering or theft.
- Creating repeatable, scalable, predictable cold chain practices and processes.
- Developing global supply chains that reach sufficient numbers of patients both for clinical trial participation and commercial viability
At Advanced Therapies Week, the CGT sector’s second annual gathering in January, I had the opportunity to share insights that support these cold chain requirements. While the CGT supply chain is daunting, another overwhelming biopharmaceutical effort – rapid worldwide distribution of the COVID-19 vaccine — offers valuable lessons for CGT, especially on the gene therapy side. COVID-19 vaccines and gene therapies need to be shipped at similar ultra-low temperature ranges (-70C to -90C) and require worldwide reach to achieve success and serve all patients in need. CSafe’s shippers and service teams have supported distribution of more than six billion doses of COVID-19 vaccine and counting.
As shared with some of the 2,000-plus CGT colleagues at Advanced Therapies Week (record attendance!), here are some of the key COVID-19 vaccine lessons that can benefit CGT:
- Develop distribution strategies early with experienced cold chain partners. In the case of COVID-19 vaccine supply chain, supply chain planning kept pace with the science.
- Engineer for supply chain deviations. In the case of the COVID-19 vaccine, the CSafe shipper — originally developed for a gene therapy — was optimized to protect the product at the requirement temperature range for up to 28 days.
- Using digital systems to ensure a tight, traceable supply chain for every shipment.
- Innovative ultra-low shipping containers, supported by highly trained service teams, can protect product security.
- With the right cold chain partner, a truly global distribution network can be built in a relatively short amount of time. In the case of the COVID-19 vaccine, that cold chain has ultimately supplied vaccine to more than 180 countries.
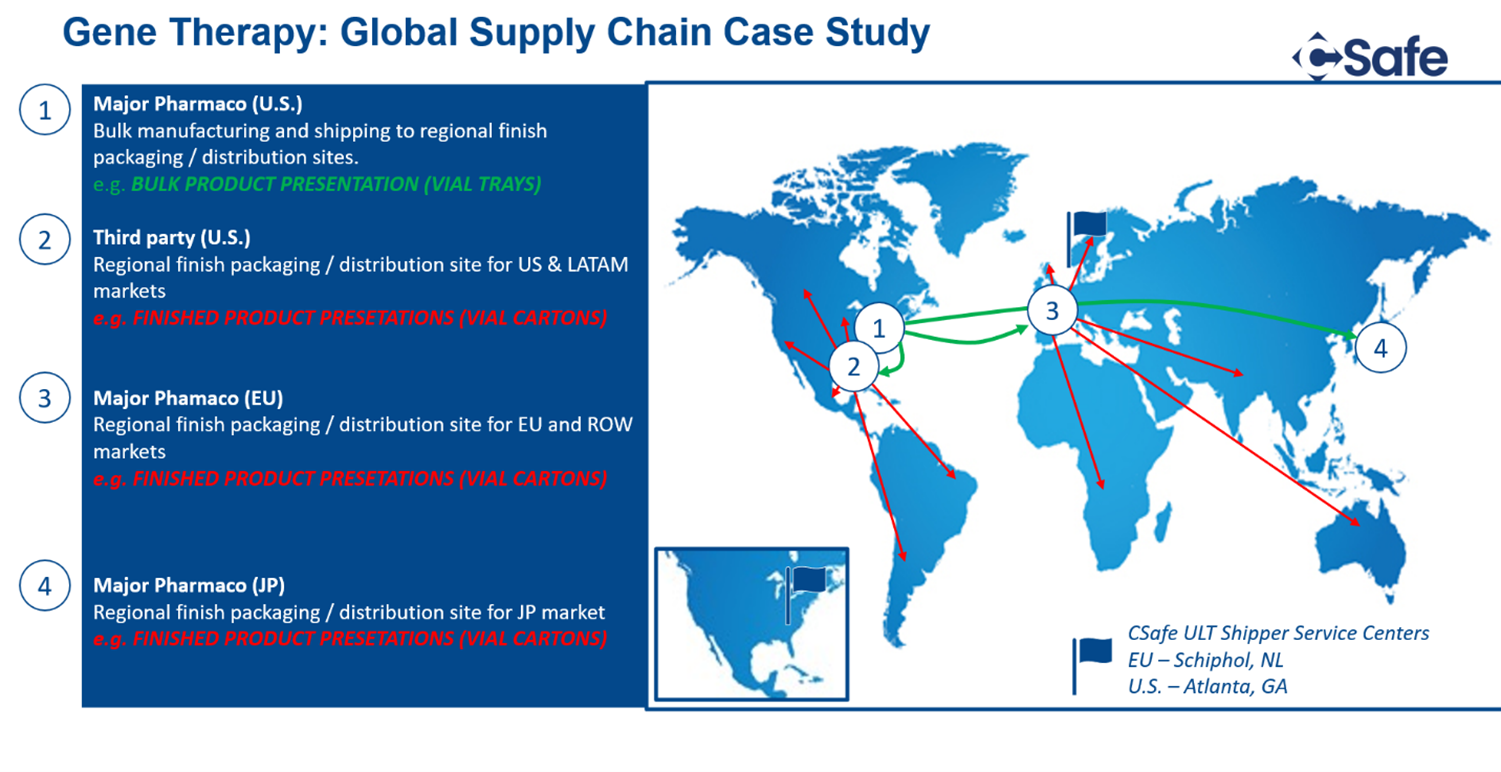 In 2023, CGT needs to take significant steps towards serving the world. Our sector simply isn’t set up to do that today. But CSafe, with more than 70 service centers worldwide, knows what is required for scale. With industry-leading shipping solutions, our here to help industrialize cell and gene therapies and make these remarkable treatments standard of care. If you have supply chain challenges, or ideas to share, please get in touch. As ARM said, in 2023, this is an opportunity we can’t miss.
In 2023, CGT needs to take significant steps towards serving the world. Our sector simply isn’t set up to do that today. But CSafe, with more than 70 service centers worldwide, knows what is required for scale. With industry-leading shipping solutions, our here to help industrialize cell and gene therapies and make these remarkable treatments standard of care. If you have supply chain challenges, or ideas to share, please get in touch. As ARM said, in 2023, this is an opportunity we can’t miss.
